Good Bonding and Coating with Contact Angle Measurements
Darren Williams answers common questions about using a contact angle measurement to determine part cleanliness. Using water to test cleanliness reveals the effectiveness of a cleaning process.
Share
Q: How do you verify that a metal part is free of surface residue that may interfere with bonding or coating?
A: Many shops use the water break test to verify that parts are clean. Water will evenly coat and “sheet off” a clean part. This test can be fooled, however, by soapy residue on the surface or soapy rinse water.
Some shops use Dyne pens to verify the surface is clean and ready for coating. Some drawbacks of Dyne pens are limited pen shelf life and a dye residue that is left on the surface, which may be undesirable for clear or thin coatings.
Every year, the Cleaning Research Group fields questions about contact angle measurements from people who are looking for inexpensive test alternatives to the water break or Dyne pen tests.
Q: What is a contact angle measurement?
A: The contact angle is a measurement of how much water “likes” the surface. It ranges from an angle of 0 degrees – complete wetting – to 180 degrees – a super-hydrophobic surface.
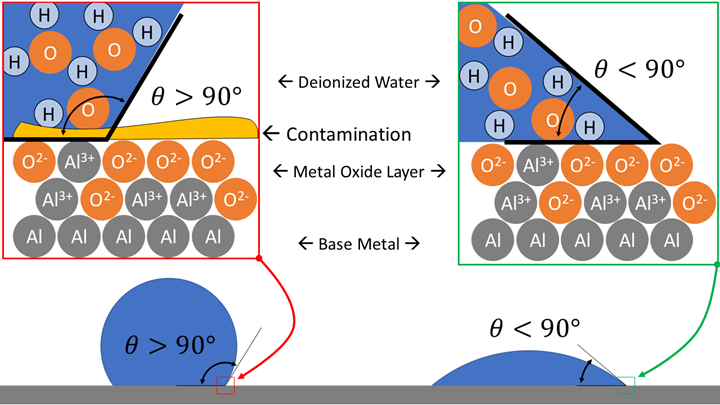
Fig. 1 – A close-up view of the contact angle of water on a contaminated (left) and a clean (right) metal surface. Photo Credits: Darren Williams
Q: How does water work to test cleanliness?
A: Nature will move toward the lowest total energy situation. The surface of metal (Figure 1) is oxidized, and this metal oxide layer is a high-energy surface. Any coating with a lower surface energy will spontaneously spread to cover the high-energy oxide layer. Pure water (for example, deionized water) has a high surface tension (72 N/m) and surface energy (72 J/m2), but still less than that of a clean and bare metal oxide surface. Therefore, a drop of pure water will spread spontaneously on a clean metal part exhibiting a small contact angle (Figure 1, right).
If any other substances (such as the organic molecules from the pine forest near our lab) come in contact with the high-energy metal oxide surface, they will spontaneously stick to the surface. These contaminations act as a coating that blocks water’s interaction with the surface. Placing pure water on the contaminated surface results in a large contact angle (Figure 1, left).
In the Cleaning Research Group Lab, we have observed the contact angle of freshly cleaned surfaces increase from 20 degrees to 40 degrees in an hour. Contact angle is an extremely sensitive surface contamination technique.
Q: Can the contact angle test be fooled?
A: Yes. If the water has a surfactant in it, then it will give artificially low results. This makes sense. Soapy water will spontaneously wet greasy surfaces because the surfactants lower the surface tension of the water (from 72 J/m2 to 20 J/m2 or so), decreasing the energetic penalty for spreading.
Pro tip: Shake the water before using it to see if the bubbles on the surface of the water last longer than expected. They should pop and disappear rapidly in clean water. Test yourself by shaking pure water and pure water with a single drop of soap in it.
Another way the water contact angle test can fail is if the metal surface has a water-loving (ionic or polar) contaminant on it. This will cause water to spread on the contaminant, making the user think the surface is clean.
Pro tip: Look for unusually shaped drops to detect this behavior. The drops often take the shape of the contamination instead of adopting a circular shape.
Q: What do I need to buy to try out water contact angle on my parts?
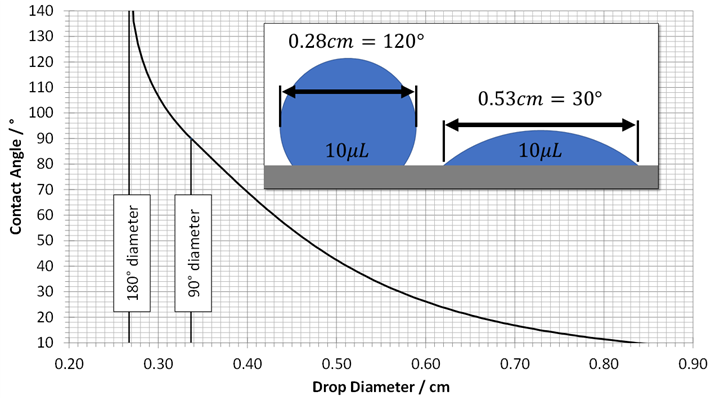
A: It is essential to use very small drops, so the water drop shape is not affected by gravity. Therefore, you should purchase a small 10-microliter pipette and some disposable pipette tips. These can be found at online retailers for under $50. The nomogram in Figure 2 is for a 10-microliter drop. This illustrates the size of the drop waist at various contact angles.
After using this for a while, a user might determine, for example, that a contact angle of less than 60 degrees consistently gives a good coating or bonding result. Then, this contact angle method as a quality check could be used. In this example, if a 10-microliter drop spreads out more than 4 mm, then the surface is clean enough to accept a good coating or bonding adhesive in the next step in the process.
For more information on this and more, visit the Cleaning Research Group online (shsu.edu/academics/chemistry/cleanresearch/) and learn about the Product Quality Cleaning Workshop at shsu.edu/pqcw.
About the Author
Darren L. Williams, Ph.D.
Williams is the leader of the Cleaning Research Group and professor of physical chemistry at Sam Houston State University. Email: williams@shsu.edu, phone: 936-294-1529.
Related Content
Sita’s CleanoSpector Measures Part Cleanliness
PMTS 2023: Handheld measuring device checks for cleanliness of parts to assure product quality as well as prior to follow-up processes.
Read MoreMultisolvent 100 Vacuum Vapor Degreaser Provides Contactless Cleaning
PMTS 2023: With applications in the most demanding industrial sectors, the machine is especially useful for cleaning parts in the turning, precision mechanics, medical and aerospace industries.
Read MoreMeeting Stringent Cleaning Goals With Modular Ultrasonic System
A knee implant manufacturer implements an advanced cleaning system that meets its tight cleaning requirements, including documenting, validating and tracing the entire cleaning process.
Read MoreIn-line Monitoring for Automated Immersion Cleaning Systems
Ecoclean’s Acoustic Performance Measurement (APM) system provides in-line measurement of ultrasonic frequency and power in fully automated immersion cleaning systems on a batch-by-batch basis or at defined time intervals, such as once a shift, day or week.
Read MoreRead Next
Test to Find Answers to a Cleaning Solution Dilemma
Finding the best cleaning fluid for a specific application is possible by carrying out practical trials, backed by lab analyses.
Read More