Meeting Medical Manufacturing’s Tough Demands
When properly applied, ultrasonic cleaning technology is well suited to play a major role in helping medical manufacturers meet their increasing need for critical cleaning.
Manufacturers in the high-visibility medical manufacturing industry are under growing scrutiny and pressure to ensure that products continue to meet ever more stringent standards for cleanliness. The push to meet these standards is coming from multiple angles.
The mention of the word “medical” brings immediate and heightened awareness to any process, especially cleaning. The life-and-death consequences of medical manufacturing tend to get attention unrivaled by that of any other industry. In a real sense, “medical” cleaning encompasses a range of industries that are all part of the overall medical manufacturing network. Suppliers, manufacturers and consumers of anything that touches the human body need to meet standards for “medical” cleaning. Historically, the major concern in medical manufacturing has been providing a product that is sterile. Until recently, cleanliness, although important for cosmetic reasons, has been of lesser consequence overall than sterility.
One problem is that the design of medical equipment seldom takes cleaning into consideration. Any random collection of medical devices will undoubtedly present a more difficult cleaning challenge than a like collection of components associated with any other field. Narrow and bent tubes, internal passages, complex linkages, tight clearances, as well as plastic and rubber components are common in medical equipment. Manufacturing processes, including sharpening, honing, polishing and others that are among the worst from the standpoint of a cleaning challenge, abound. Most devices, although their sub-components may be cleaned several times in the manufacturing process, require a final cleaning in their assembled state—sometimes with guards, safety devices and even closures in place. In short, the equation couldn’t be worse! Cleaning challenges in the medical industry are, without question, among the most difficult faced by the cleaning community.
The importance of cleaning in the medical industries has recently been elevated by the growing recognition that sterility alone does not assure product safety. Attention has recently focused on the fact that residuals of even deactivated biological entities pose a long-term threat to patients if these residues aren’t removed from items such as body implants. Sterilization, however, is frequently performed after “final” cleaning, leaving the possibility that potentially harmful biological residues, known as “bioburden,” remain.
Medical cleaning requires the removal of a range of contaminants. Solid contaminants may be large, small, microscopic, or, in some cases, even sub-microscopic, and come in every shape that can be imagined. They may be as hard as diamonds or as soft as rubber. Some are soluble, while others are partially or totally insoluble. The forces holding contaminants in place, likewise, range from the contaminants being physically imbedded in the substrate to simple molecular adhesion. Entrapment in complex geometries and capillary spaces is common, since many devices are cleaned after assembly.
Assuring cleanliness is further complicated by the fact that many contaminants relevant in medical applications are, at best, as difficult to detect as they are to remove. Cleanliness evaluation may require complex laboratory techniques that may take hours or days to perform, making real-time process validation difficult to impose. Process verification lends itself well to the validation of sterilization processes using heat, chemicals and radiation. Process verification methods often do not, however, adequately validate cleaning processes. They tend to lead to a false sense of security unless cleaning procedures are totally proven and, frequently, to error on the side of the extreme.
Things are changing rapidly in the world of cleaning for medical applications. Specifications are still evolving and, unfortunately, appear to be on several diverse tracks. At this writing, no universal specification exists that, if met by any manufacturer, can give complete comfort and assurance that all requirements for medical cleanliness have been met.
The Challenge
Because of the range of geometries, construction materials and contaminants involved, and the critical requirements associated with it, cleaning for medical applications presents a challenge not found in other industries and often not easily answered by conventional off-the-shelf technology. Thorough and reliable cleaning for medical applications may require a combination of technologies, each not up to the challenge on its own, brought together in specialized equipment. Frequently, the most successful formula includes ultrasonics.
Ultrasonic technology is a natural fit because many cleaning specifications use ultrasonics to extract residual contaminants for metrological examination. By doing so, these methods recognize the superior effect that ultrasonic cleaning is able to achieve.
Almost a century after it was first used for cleaning, ultrasonic technology continues to evolve at an increasingly rapid pace. Many recent developments position ultrasonics as an ideal and sometimes the only answer to many of the challenges found in cleaning for the medical industry.
Sweeping Frequency
Ultrasonic transducers are designed to operate at a discreet frequency or frequencies defined by their physical dimensions. Operation at a fixed frequency, however, can result in non-uniform intensity of the ultrasonic field in an ultrasonic cleaning tank because of interaction and reflection of the sound waves. Advances in both transducer and generator technology now allow ultrasonic frequencies to warble or “sweep” around a central frequency. The result is improved distribution of ultrasonic energy and improved cleaning efficiency overall.
This method, effective as it is, on rare occasion has been associated with damage to delicate assemblies sensitive to the sweeping frequency. The introduction of non-uniform sweep, continuously varying the frequency of the frequency sweep or “warble,” has eliminated this potential stumbling block.
Increased Ultrasonic Frequency
Significant research has shown that different cleaning requirements are ideally met using different ultrasonic frequencies. Higher ultrasonic frequencies, for example, remove smaller-sized particles more efficiently than lower frequencies. The range of ultrasonic frequencies continues to increase and now has reached almost 300 kHz.
Multiple Ultrasonic Frequencies
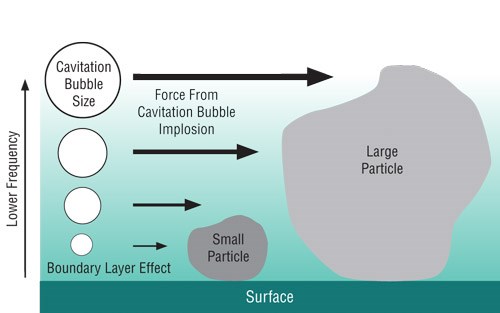
Cavitation bubbles provide the primary mechanism of ultrasonic cleaning. The formation and energetic collapse of these bubbles on a scale that allows their effect to interact with contaminants is necessary for the process to work. Higher frequency ultrasonic sources create more, but smaller and less energetic cavitation bubbles, while lower frequencies provide fewer, but larger and more energetic cavitation bubbles.
Although higher frequencies excel at removing smaller particles, lower frequencies may be required to remove particles of a larger size. Since cleaning often requires the removal of contaminants having a range of particle sizes, the use of several frequencies in succession is ideal. Similarly, some ultrasonic frequencies outshine others in their ability to facilitate and promote physical phenomena such as dissolution and emulsification, as well as chemical reactions such as those required for passivation. Recent research has further verified that multiple frequency ultrasonic excitation can also enhance the ability of ultrasonic energy to penetrate into increasingly smaller geometries to facilitate contaminant removal.
Until fairly recently, applying different frequencies meant separate ultrasonic cleaning tanks. Now, however, it is possible to apply multiple frequencies in a single tank. Frequencies may be applied sequentially at varying intensity and in any order, depending on the range of particle sizes and the other contaminants being removed.
Future Promise
Ultrasonic energy shows early promise in aiding in the sterilization of some critical medical components. Ultrasonic cavitation and the subsequent violent collapse of the cavitation bubble has been demonstrated to be at least partially effective at breaking down cell walls and causing other effects that, at minimum, speed or enhance other sterilization schemes. The addition of ultrasonics may reduce the required temperatures and chemical concentrations, but this research is ongoing.
Unique Capabilities and Shortcomings
To support its value in medical and medical-related cleaning, ultrasonics brings unique capabilities that cannot be provided by other technologies: enhanced small particle removal; the ability to penetrate enclosed spaces; non-line-of-site capability; and easy integration with other technologies.
For all of its capabilities, ultrasonic cleaning also has inherent limitations that must be understood. Mechanical vibration is the mechanism that is at the base of all ultrasonic cleaning processes. This mechanical vibration, powerful in its effect, requires a suitable means of transmission for delivery to the cleaning site. This means of delivery is a liquid. All surfaces of parts that are to be cleaned must be immersed in this liquid. Likewise, the liquid must support the formation and energetic collapse of cavitation bubbles as mentioned earlier. Water, and mixtures of water and certain chemistries provide excellent vehicles for ultrasonic cleaning. Anything that provides a “disconnect” in the liquid path will likely prove detrimental to ultrasonic cleaning.
Disconnects include liquids that do not support cavitation (some solvents for example), pockets of trapped air, and baskets or fixtures that are massive or are constructed of “soft” materials, including plastic or rubber. Overloading an ultrasonic cleaning tank may also compromise the ultrasonic cleaning effect. For similar reasons, parts fabricated totally or in part of rubber-like materials and some forms of plastic, may not be good candidates for ultrasonic cleaning.
Successful Ultrasonic Cleaning
Experienced manufacturers of ultrasonic equipment are the best resource for assistance in developing an ultrasonic cleaning process. The technology, although not “rocket science,” has occasionally shown itself to be somewhat non-intuitive and has frequently been misapplied by those with limited or no experience. Test cleaning using equipment as close as possible in both configuration and scope to that proposed-for production is highly recommended.
The overall process usually involves more than a simple ultrasonic cleaning tank. To be effective and productive, an ultrasonic cleaning system needs to address maintenance of bath quality through filtration or periodic replenishment, rinsing methods for removing cleaning residues, and part drying. Automated material handling, process monitoring and a number of other features also need to be considered in the overall process design.
The Big Picture
Cleaning requirements for the medical industry are in flux. Specifications that are now being developed, when applied, will almost certainly force a change in the way medical manufacturers clean things. This highly visible industry will be held more and more responsible and accountable for its cleaning processes. Ultrasonic technology, when smartly and properly applied, can enhance many cleaning processes.
Related Content
Sita’s CleanoSpector Measures Part Cleanliness
PMTS 2023: Handheld measuring device checks for cleanliness of parts to assure product quality as well as prior to follow-up processes.
Read MoreCorrosion Prevention: How to Avoid the Enemy of Metal Parts
This chemical reaction that is a constant, indiscriminate and costly enemy of metal parts is preventable, but intentional measures must be taken and become an essential process within a company’s walls.
Read MoreParts Cleaning Sector Shifts Energy Toward Regulatory Changes
With changes in EPA regulations regarding the use of some popular cleaning fluids, cleaning suppliers and end users are readjusting business strategies and/or cleaning processes to meet new requirements.
Read MoreMeeting Stringent Cleaning Goals With Modular Ultrasonic System
A knee implant manufacturer implements an advanced cleaning system that meets its tight cleaning requirements, including documenting, validating and tracing the entire cleaning process.
Read MoreRead Next
Aqueous Cleaning for Aerospace
A turbine manufacturing plant phases out an obsolete vapor degreasing system, making the change to aqueous-based cleaning.
Read MoreA Tooling Workshop Worth a Visit
Marubeni Citizen-Cincom’s tooling and accessory workshop offers a chance to learn more about ancillary devices that can boost machining efficiency and capability.
Read MoreSeeing Automated Workpiece Measurement in Real Time
User-friendly inspection software for CNC machining centers was shown at IMTS 2024 monitoring measurements between and after machining while performing SPC based on recorded measurement values.
Read More