3 Common Filtration Questions Answered
Learn about the variety of filters for removing particulates from a cleaning fluid, how to determine cleaning fluid life and more.
Filtration is the mechanical process to separate unwanted materials from a cleaning fluid (cleaner) during a cleaning process. The filtration unit is a medium through which only the cleaner can pass. In a direct, continuous and expedient way, filtration removes fine particulates, metal shavings, oils and grease from the cleaner bath. Polypropylene, modified papers, organic and related hydrocarbon products are the most effective media for removing contaminants in a cleaner.
Although the concept is straightforward, manufacturers still have questions about various aspects and options when it comes to cleaning fluid filtration. Here, Hubbard-Hall Inc. (which develops, services and supplies a line of specialty chemicals for ferrous and non-ferrous metals) offers answers to three common filtration questions to enable manufacturers to determine the best filtration processes for their specific applications.
1) What types of filtration options are available?
When filtering a cleaner, there are a variety of filter types to choose from. The type chosen will depend on the filtering requirements for a particular application. Removing oils, grease, shop dirt and metal fines extend the practical service life of the cleaner as well as maintain optimum hard surface cleaning of metal parts, preparing the surface for subsequent treatments.
Membrane Filtration. This technology uses a permeable membrane system where the soiled cleaner is pumped through the membrane tubes. Molecules of sizes larger than the components of the cleaner such as oil and coolant are blocked and diverted to a discharge. The aqueous cleaner passes through and returns to the process tank. Membrane filtration provides a rapid and dramatic filtering action and can reclaim more than 98% of the cleaner. Membrane filtration is most suitable with soak/emulsifying cleaners.

The media of choice to remove contaminants in a cleaner consists of polypropylene, modified papers, organic and related hydrocarbon products. Shown is a paper-style disc filtration unit. Photo Credits: Hubbard-Hall Inc.
Cartridge Filters. These enclosed canister types have a polypropylene center around which similar fiber material is tightly wound. The porosity of the filter medium can range from 100 microns to below 5 microns, based on the specific filtering requirement. Particles are retained in the media pattern, and the material absorbs oily solutions as well.
The cleaner is continually pumped through the filter cartridge. It is a relatively simple yet effective system to remove the typical contaminants found in the cleaner, and it does not take up much floor space. When spent, the supplier can often dispose of cartridges directly to a certified destruct facility.
Oil-Absorbing Filters. These units consist of an enclosed housing that contains polypropylene baskets with special oil-absorbing, plastic-type media. The cleaner is pumped through the enclosed system, where the media absorbs oils and grease. The saturated media is replaced as needed.
Bag and Indexing Fabric Filters. The cleaner is pumped through a large filter chamber, where oil, grease and particles are retained. However, this filtration system is not suitable for systems cleaning large volumes of very oily parts, and it consumes a large amount of floor space.
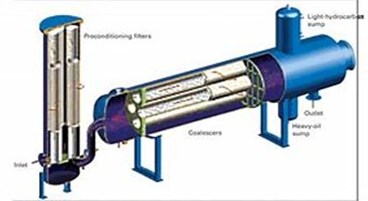
A coalescer channels the flow of the cleaner, separating the aqueous from the oily solution.
Coalescer. A coalescer channels the flow of the cleaner, separating the aqueous from the oily solution.
Belt & Disc Filters. These devices efficiently remove displaced, floating oils; take up minimal space; plug into a 110-V outlet; and operate automatically. Both filters are typically installed in a side tank where the solution cools sufficiently

to release oils, to be removed by either the belt or disc.
2) How does one determine cleaning fluid life? The ideal condition for any cleaner is the freshly prepared working solution, right before the immersion of any parts. Following the first part load, the cleaner

The belt skimmer (top) and disc skimmer efficiently remove displaced floating oils; take up minimal space; plug into a 110-V outlet; and operate automatically. Both filters are typically installed in a side tank where the solution cools sufficiently.
bath becomes soiled, as it should if it is the right soak cleaner for the job.
Production continues and, as it does, the bath steadily loads up with contaminants. These typically consist of oils, grease, shop dirt and fine particles. Telltale signs of the contaminated cleaner become more visible: the solution turns either a dark or tea brown-to-milky color; oils tend to separate and float (especially as the cleaner cools); grease rings form on the walls of the tank; and sludge and particles build up on the bottom of the tank.
How soiled does the cleaner have to get before it becomes a problem? And when is it that maintenance additions of the cleaner do not improve cleaning performance?
Suppliers will provide a simple, effective analysis procedure to evaluate cleaner strength. The majority of soak cleaners — over 90% — are of the alkaline type. The analysis is, in effect, a chemical neutralization, adding a standard acid to an indicator color change. Using a calculation per given product factor, the concentration of active product in the soak cleaner bath is determined. Appropriate additions of product concentrate are made, based on the analysis results, adjusting product concentration to a targeted value.
The problem is that, over time, additions made in this manner gradually widen the gap between corrective and practical. The gap referred to is the “worsening effect” that contaminants exhibit, thus negating the intended practical cleaning improvements that product additions are intended to make. All cleaners become exhausted at some point in their service life. This means that the titration analysis has a practical limit. That said, there are simple, quick and effective test procedures that can provide important cleaner bath data, complementing the titration or test kit procedure.
A few quick tests might alert the user to potential trouble — and, therefore, help avoid a problem entirely. The focus of these tests not only provides a more quantitative analysis of the cleaner bath but can also help plan a dump and new makeup to avoid interrupting the production schedule. More importantly, the goal is to eliminate finishing rejects due to poor hard surface cleaning. Here are a few tips.
• Specific Gravity. Measure the cleaner bath’s specific gravity when first made up (no parts yet immersed). On a regular schedule, measure and record the specific gravity as the bath ages, while making sure to maintain the cleaner concentration at the initial makeup. There will be a point at which the data coincides with a drop in quality cleaning. The specific gravity will have increased to a point that indicates the amount of oil, grease, shop dirt and particles that have built up in the bath. Corrective action may include making additions of the cleaner concentrate, cutting the bath and replenishing, or dumping and replacing with a new makeup.
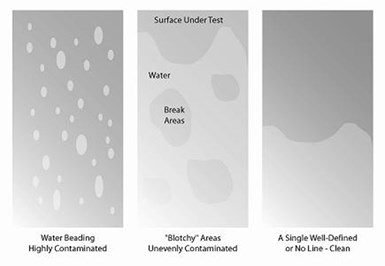
After cleaning a surface, a performance test can be done to determine if the parts were cleaned efficiently. Water beading or blotchy areas mean that contamination is still on the surface.
• Performance Test. Immerse a clean panel (for example, a steel hull cell panel) in the cleaner bath at the same temperature and amount of time as the parts. Rinse in cold running water for 60 seconds and examine for water breaks. Next, immerse in dilute acid (5% hydrochloric or sulfuric acid) for 15 seconds, followed by rinsing in cold running water. Examine for water breaks. A positive observation of water breaks at either step indicates deposition of soils from the cleaner bath onto the panel. Corrective action may include making additions of the cleaner concentrate, cutting the bath and replenishing, or dumping and replacing with a new makeup. If adding 15-20% of the initial cleaner makeup does not eliminate the water breaks on retest, dump the cleaner and replace it with a new makeup.
Water breaks, as seen in this image, are a sign that contamination is still present on a surface. But during a performance test, a surface is clean when a single, well-defined line or no line is visible.
• Oil Displacement. First, conduct the described test on a sample of the newly prepared cleaner that has not been used.
As the bath ages, the concentration of emulsified oily soils becomes more concentrated. Take a 90-mm sample of the hot cleaner, and slowly and carefully add 10 mm of 10% sulfuric acid. Mix the solution well for about 15 minutes. Then, quickly and carefully pour the solution into a clean 100-mm graduate cylinder; adjust volume, if necessary, with a small volume of water up to 100 mm. Observe as the oily soils separate. The direct read is the percent of displaced oils before the correction factor. Additionally, take note of any heavier sludge that may have settled. These are also contaminants that may be a combination of mineral or chlorinated paraffinic oils (heavier than water) and other sludge. Compare the percent of displacements with the results of the freshly prepared cleaner. Any percent of displacements for the new cleaner can be used as a correction factor when measuring percentage displacements in the aged soak cleaner.
(milliliters of displaced oils in aged soak cleaner) – (milliliters of displacement reading in new cleaner) = direct percentage of displaced oils in aged cleaner.
There are some instances where a buildup of metallic contaminants may result in smut formation on parts that should be cleaned.
It is important to conduct the oil splitting test on the new cleaner, as the acid reaction may split out a discernable volume of surfactants and wetting agents. If there is no split in the new cleaner, there is then no correction factor.
Corrective action should now sound familiar, making additions of the cleaner concentrate, cutting the bath and replenishing, or dumping and replacing with a new makeup.
There are some instances where a buildup of metallic contaminants may result in smut formation on parts that should be cleaned. Or, such metal contaminants may be complex with the detergency complement of the cleaner, thus reducing the hard surface cleaning effectiveness. Shops that have an atomic absorption spectrophotometer (AA) can use the instrument to analyze for such critical metals that may result in high iron, zinc or copper particle counts, such as:
copper and zinc: 300-500 ppm
iron: 800-1,000 ppm
Control examples like these confirm or predict the point at which the cleaner — even with proper maintenance additions and at the correct operating temperature — will approach its maximum service life. In most instances, adding more cleaner concentrate will restore quality cleaning, but perhaps only for a short time, as these practical tests indicate.
3) What are the benefits of fluid filtration? Consider the following benefits that accrue — with cost savings — by filtering the cleaner and testing for practical service life:
- Extending cleaner bath service life means less downtime and longer periods of uninterrupted productivity.
- Fewer bath dumps reduce the waste-treatment workload.
- Minimizing contaminants helps to maintain solution consistency closer to new makeup.
- Quality cleaning results in proper surface preparation, which leads to quality finishing and posttreatments.
In addition, filtration can be supplemented by the application of mechanical oil removal devices that are cost-effective and can be used in-tank.
An overflow weir or side tank can collect cleaner solution (which cools down about 10-20°F below the temperature of the cleaner) while oils separate to the top. The oils can be skimmed off using a disk or belt.
Hubbard-Hall Inc. | hubbardhall.com
Related Content
Kyzen Solvents Provide Safe Parts Cleaning
The SLV901 and SLV803 solvents are formulated to maintain cleaning efficacy while providing a safe, environmentally friendly alternative to processes that use PFAS and HFCs.
Read MoreVersatile Sandblasting for Deburring Intricate Geometries
PMTS 2023: Comco’s MicroBlasting sandblasting systems can deburr, texture and clean small, intricate parts.
Read MoreMeeting Stringent Cleaning Goals With Modular Ultrasonic System
A knee implant manufacturer implements an advanced cleaning system that meets its tight cleaning requirements, including documenting, validating and tracing the entire cleaning process.
Read MoreIn-line Monitoring for Automated Immersion Cleaning Systems
Ecoclean’s Acoustic Performance Measurement (APM) system provides in-line measurement of ultrasonic frequency and power in fully automated immersion cleaning systems on a batch-by-batch basis or at defined time intervals, such as once a shift, day or week.
Read MoreRead Next
Removing Particulate Contaminants from a Cleaning System
Employing the most appropriate technique and a correctly designed system for maintaining bath quality can lower cleaning costs.
Read MoreA Tooling Workshop Worth a Visit
Marubeni Citizen-Cincom’s tooling and accessory workshop offers a chance to learn more about ancillary devices that can boost machining efficiency and capability.
Read More