Preventing Metal Corrosion with Metalworking Fluids
All manufacturers deal with metal corrosion and rust. Learn about the causes of metal corrosion and options for preventing it.

Rust is a type of corrosion that occurs on iron and steel parts. Both ferrous and non-ferrous parts are vulnerable to corrosion.
Heat removal is one of the most important functions of a metalworking fluid. Effective heat removal yields good tool life and dimensional accuracy of parts. Water has a greater capacity for removing heat than oil; however, water alone in contact with freshly machined metal leads to corrosion. Thus, corrosion is a problem faced by every user—and manufacturer—of water diluted metalworking fluids. However, corrosion can occur even with dry cutting and is not simply due to the use of water-based fluids.
Seasonal Corrosion
Corrosion can occur at any time during the year, but normally it occurs more often during July, August and September when temperatures and the relative humidity are high. This applies to the Eastern and Midwestern areas of the United States. Corrosion is not usually as much of a problem in arid states, such as Colorado, New Mexico, Arizona, Utah and California, because the relative humidity is usually low.
As temperature increases, the rate of all chemical reactions also increases. This includes corrosion. High temperature in the presence of moisture and oxygen in the atmosphere is the reason corrosion happens more in the summertime.
Moisture condenses on a part and acts as an electrolyte to form a galvanic cell. If the concentration of the fluid, which provided rust protection during the fall and winter months, does not provide protection when the humidity climbs, an adjustment in the fluid concentration is necessary. If the concentration of 1:30 (3.3 percent) was adequate during fall and winter, then the concentration may need to be increased to 1:25 (4 percent), or to the point where rust is no longer seen. If a fluid user objects to increasing the concentration of his central system mix for reasons such as foam and potential skin problems, it may be necessary to increase rust protection with the use of additives. The additives used depend upon the type of metal(s) involved, the user’s chemical restrictions, additive availability and the fluid used.
pH and Corrosion
The pH of a metalworking fluid is a factor in controlling corrosion. A high pH, greater than 9, will protect ferrous metals but will adversely affect the corrosion control of non-ferrous metals, such as aluminum, brass and bronze.
When the pH is low in an individual machine, the easiest solution to the problem is to dump, clean and recharge with a new mix of the fluid product at the recommended concentration. If treating a central system mix, which is being used on ferrous metals, adjust the pH to between 8.8 and 9.2 with the proper additives. Excessively high mix pHs are usually a sign of contamination, and the mix should be dumped and recharged.
If non-ferrous metals are being machined, and staining or pitting is a problem, check the product information to determine if the product was designed for use with non-ferrous metals.
Water’s Effects on Corrosion
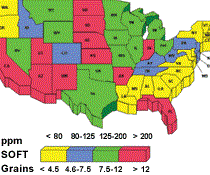
Water hardness affects cutting fluid balance. Different geographic regions have different degrees of water hardness. Compensating for water hardness will optimize fluid performance.
Chemicals in the water used to make up and maintain the mixes can increase the rate of corrosion. All water contains ions, some of which are aggressive and can cause corrosion of most metals.
Waters that contain more than 100 ppm chloride, more than 100 ppm sulfate, or 50 ppm nitrate are considered aggressive waters. Chlorides, sulfates and nitrates cause corrosion by breaking down the protective barriers on the surface of the metal, opening the way to corrosion. Continuous addition of water increases the sulfate, chloride, and nitrate content of the water in a central system, thus making it more aggressive the longer it is used.
If aggressive water is suspected, take a sample of the water and have it completely analyzed. When a central system fluid is suspected to cause corrosion, submit a sample of the mix for ion determination. If the chloride, sulfate or nitrate content is higher than the acceptable limits, the user may have to change sources of water. It may become necessary, for example, to change to a blend of deionized or distilled water with the regular water. A change to another metalworking fluid with superior corrosion control may also be an answer.
Many desirable properties of metalworking fluids literally can be destroyed by chemical reactions with dissolved water solids. The most familiar example of this is the effect of “water hardness,” which is essentially the calcium and magnesium content of the water.
The divalent ions react with soaps, wetting agents and emulsifiers to form compounds with limited solubility. The formation of these insolubles depletes rust inhibitors, which results in rusty parts and machines. Hard water is any water containing greater than 250 ppm calcium carbonate or 15 ‘grains’ hardness. The higher the hardness is, the more prone the fluid is to corrosion.
Conductivity is another means of determining the number of dissolved ions in the mix. Higher conductance promotes corrosion, mix instability, residue and other problems. Conductivities of greater than 4 MilliSiemens per cm are considered high.
Metal Corrosion Caused by Bacteria
High bacteria counts in the fluid mix can lead to corrosion. Bacteria consume metalworking fluid components, and the by-products of this activity can lower the mix pH. The metabolic products include mild organic acids that lower the pH of the fluid and, therefore, the corrosion resistance of the fluid is compromised. Also, if left unchecked, bacteria can split emulsions.
If an individual machine’s mix has a high bacteria count, the easiest solution to the problem is to dump the old fluid, clean the sump with a good machine cleaner, rinse with fresh water, and then recharge with a new fluid mix at the recommended concentration. If it is impractical to dump the charge, treat it with an appropriate microbicide. In a central system, use preventive measures including the correct use of microbicides to prevent and control bacterial growth.
Lean Metalworking Fluid Concentration
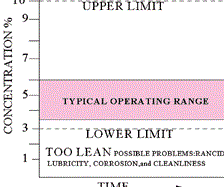
Every metalworking fluid formula has a “sweet spot” where the concentration should be maintained. Checking the concentration frequently will prevent performance and environmental problems.
Ingredients in a metalworking fluid are designed to be effective within a specified dilution range. If the fluid becomes leaner than the specified range, the leaned-out ingredients may not be able to perform their designed jobs. This also applies to the rust inhibitors, which may not be able to protect the new ground or machined parts from corrosion.
Check fluid concentration frequently and routinely. Titration methods and refractometers are the best methods of concentration determination. Add concentrate to obtain the recommended dilution. In individual machines, do not attempt to balance the fluid with the use of additives. Dump and recharge.
Extended In-Process Time
Normal in-process rust protection provided the metalworking fluid product is used at the recommended concentration, is up to 72 hours. Most fluid products provide in-process rust control for a variety of metals from high alloy steels to cast iron. At the proper concentration, it is reasonable to expect 48 hours of protection for cast iron and 72 hours for high alloy steels. If corrosion protection beyond these time limits is required, then the use of a longer-term rust preventive is recommended.
Bimetallic Corrosion
Bimetallic corrosion is the corrosion of two different metals in contact with each other and occurs when a transfer of electrons from one metal to another takes place with the cutting fluid acting as a conductor. The solving of bimetallic corrosion problems is a matter of getting a nonconductive material between the two different metals. This may require switching to a fluid that has better bimetallic corrosion prevention properties. In general, this would be a fluid containing a high oil content, such as a premium soluble oil. Waterproof greases or a thin plastic sheet can also be used between the contacting surfaces.
Broken Emulsion
Rust can happen if an emulsion breaks, in which case the instability of the emulsion will make the rust inhibitors ineffective. If the emulsion appears watery and the sample shows stratification, the emulsion may be unstable or broken. To solve this problem in individual machines, the best solution would be to dump, clean the machine, rinse with fresh water, and recharge with a metalworking fluid mix at the recommended concentration.
Broken and unstable emulsions are often caused by hard excessive bacterial contamination. It is important to determine the cause of the broken emulsion before taking corrective action. When dealing with a central system, it may be possible to use an additive that is an emulsifier to re-emulsify the product. If time permits, send samples to the fluid supplier for tests. If time does not permit, then run jar tests on-site to determine what additives to use and the proposed dilution ratio.
Process Procedures
Parts sometimes corrode when stacked one on top of the other or when contacting each other in tote bins. This is caused by the fluid acting as an electrolyte and forming a galvanic cell between the two parts. This happens very readily with cast iron. In fact, with stacked cast iron (depending upon the quality iron) it is sometimes difficult to control rust for more than several hours.
Placing a fiber spacer between the stacked layers of parts can eliminate this condition. Do not use cardboard as the spacer because of its high content of sulfur. If using paper or similar materials, be sure they are the vapor phase inhibiting (VPI) type, or they could promote rust. Enclosed tote bins retain moisture and promote corrosion. Use a wire basket for parts or drill holes in the bottom of the tote bins to drain the fluid. If possible, use plastic tote pans or plastic-covered wire baskets.
Fingerprint Corrosion
Parts sometimes corrode after being touched by human hands. Material handling can cause corrosion in the design of fingerprints in the newly machined surfaces of the metal parts. This is more prevalent among people with more acidic skin conditions and on highly finished parts with low Ra finishes. The use of a fingerprint neutralizing rust preventive usually will correct such fingerprint corrosion.
Dirt Recirculation
Small metal particles in a metalworking fluid are sometimes referred to as "dirt" or "swarf." Swarf deposited on the part and not properly washed away forms a galvanic cell, and rust will occur underneath the swarf. Correct the dirt recirculation in an individual machine by having the machine dumped, cleaned, rinsed with fresh water, and charged with a fresh fluid mix at the recommended concentration.
Spontaneous Combustion
Spontaneous combustion is the ignition of substances apparently without any direct cause. When large quantities of metal fines become soaked with relatively small amounts of oils and are exposed to air, they may ignite spontaneously if conditions are just right. The presence of moisture frequently aids spontaneous combustion, and at times steam may be seen rising from piles of metal chips, a harbinger that such combustion may occur.
Heat can be produced by the reaction between newly cut, damp chips and cause a fire to start if enough organic material, such as oil, is present. The newly cut chips expose a nascent metal surface that is reactive under certain conditions. Chips of the same material form heat by oxidation; non-like materials can form hydrogen gas, which—if confined (in the chip pile)—can smolder and even ignite. The type of metalworking fluid used can help aid or retard these reactions.
Here are some steps that will help prevent spontaneous combustion from the chips. Reduce or eliminate the amount of metalworking fluid remaining on the chips by blowing the chips dry (if possible) or by centrifuging. Spread the chips over a large area or circulate air throughout the chip/swarf pile. The use of a metalworking fluid with a high mineral oil content can reduce the likelihood of spontaneous combustion, or one can adjust the concentration richer if a soluble oil type fluid is already in use. Spraying the chip pile with a water-based corrosion inhibitor may also be helpful.
In a central system, dirt recirculation could mean a malfunction of the filter. To help settle swarf, select the settling aid or clarifying agent compatible with the fluid and filter system. Check the pressure at the coolant nozzle to determine if the lack of coolant pressure is causing the swarf to build upon the fixtures of the machine and the workplace. The normal coolant pressure is 20 psi.
Corrosive Atmospheres
If corrosion occurs after the metal removal operation and while the parts are being stored, the cause may be a corrosive atmosphere. Heat treat departments may exhaust corrosive fumes. One of the most corrosive is sulfur dioxide fumes, which will quickly corrode metal surfaces. Cement dust from construction work will also quickly corrode metal surfaces.
Ventilation of the metal removal area is the only answer to corrosive fumes. Get rid of the fumes, and you get rid of the rust.
Related Content
The Case for Higher-Performing Metalworking Fluids
Machine shops have the opportunity to enhance their profitability by choosing the proper metalworking fluids for each machining application.
Read MoreAutomate Coolant Management, Collect Data to Optimize Machining Operations
The future of coolant management lies in better control through automation and applying real-time data analysis and insights.
Read MoreMaster Fluid Solutions Semisynthetic Coolant Improves Sump Life
Trim MicroSol 685XT is designed to provide enhanced corrosion inhibition on all ferrous and nonferrous metals.
Read MoreAutomating Cutting Fluid Management
Automation can amplify the benefits of cutting fluid management while reducing the maintenance burden on shopfloor employees.
Read MoreRead Next
Cleaning and Corrosion Protection with Solvents
Protect parts against rust in an efficient and sustainable way.
Read MoreMaximizing Cleaning and Rust Preventive Programs
Properly managed and controlled chemistry applied to the correctly designed aqueous parts washer is a formula for successful cleaning and corrosion resistance.
Read More5 Aspects of PMTS I Appreciate
The three-day edition of the 2025 Precision Machining Technology Show kicks off at the start of April. I’ll be there, and here are some reasons why.
Read More





















.jpg;maxWidth=970;quality=90)